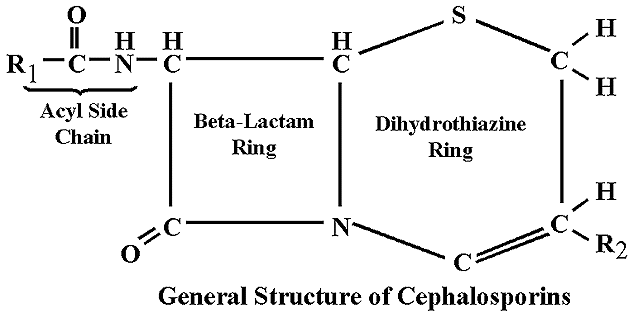
Beta-lactamase is any member of a group of enzymes which break down penicillins and cephalosporins and destroy their antibiotic activity. These enzymes are produced by gram-negative bacteria, and allow them to be antibiotic resistant. The following image summarizes the effects of beta-lactamase on penicillin:
This is the structure of the Beta-lactamase enzyme itself:
Here is another rendering of the molecule, from the bacteria Francissela tularensis, the inhibitor at the active site of the molecule, is shown in blue.
Here is a related structure in a bacteria that is commonly known, Escherichia coli, once again, the inhibiting acid at the active site is shown in blue:
In this drawing the structure of the active site can be clearly determined, including the 6-carbon ring.
Beta-lactamase is used as a catalyst for the hydrolysis of depsipeptides. This allows the beta-lactamase to destroy the beta-lactam ring in penicillins and cephalosporins.
The reactions of beta-lactamase do not have to be catalyzed, and occur in the presence of any beta-lactam ring. Beta-lactamase consists of a series of 265 amino acids, and the Protein Data Bank reported the molecular/structural weight to be 30077.55 but does not give the units.
Sources:
Medical Dictionary
Photo #1
Photo #2
Photo #3
Structures
Reaction Info.
Photo #4
Protein Data Bank
Thursday, March 14, 2013
Friday, February 8, 2013
Tryptophan
Recently, we have been discussing organic molecules, and each student in the class chose an organic molecule to research. I chose tryptophan.
Many of you may know tryptophan as "the thing that makes you sleepy when you eat turkey."
However, tryptophan looks more like this,
Than this:
In fact, the correct (IUPAC) name for tryptophan is (2S)-2-amino-3-(1H-indol-3-yl)propanoic acid. (And I thought tryptophan was a mouth-full!) Tryptophan is one of the 20 different kinds of amino acids (monomers that combine to form proteins). It is also an essential amino acid, which means it can't be synthesized in the body and must be consumed (in forms like turkey).
The chemical formula: C11H12N2O2 gives tryptophan a molecular weight of a whopping 204.225 grams per mole! Tryptophan is soluble in water, boils at a temperature of 447.908 °C, and melts at 280-285 °C. Tryptophan also contains hydrogen bonds, which means that there are also dispersion and dipole-dipole intermolecular forces.
So maybe, the next time you eat a bite of this:
Many of you may know tryptophan as "the thing that makes you sleepy when you eat turkey."
However, tryptophan looks more like this,
In fact, the correct (IUPAC) name for tryptophan is (2S)-2-amino-3-(1H-indol-3-yl)propanoic acid. (And I thought tryptophan was a mouth-full!) Tryptophan is one of the 20 different kinds of amino acids (monomers that combine to form proteins). It is also an essential amino acid, which means it can't be synthesized in the body and must be consumed (in forms like turkey).
 |
| Good news for this guy, huh? |
The chemical formula: C11H12N2O2 gives tryptophan a molecular weight of a whopping 204.225 grams per mole! Tryptophan is soluble in water, boils at a temperature of 447.908 °C, and melts at 280-285 °C. Tryptophan also contains hydrogen bonds, which means that there are also dispersion and dipole-dipole intermolecular forces.
So maybe, the next time you eat a bite of this:
You'll think more about our friend, tryptophan, than "just the stuff that makes you sleepy."
If you want to learn more about tryptophan, this was the source I used. The same website has a link to a 3D molecular structure!
Subscribe to:
Posts (Atom)