Beta-lactamase is any member of a group of enzymes which break down penicillins and cephalosporins and destroy their antibiotic activity. These enzymes are produced by gram-negative bacteria, and allow them to be antibiotic resistant. The following image summarizes the effects of beta-lactamase on penicillin:
This is the structure of the Beta-lactamase enzyme itself:
Here is another rendering of the molecule, from the bacteria Francissela tularensis, the inhibitor at the active site of the molecule, is shown in blue.
Here is a related structure in a bacteria that is commonly known, Escherichia coli, once again, the inhibiting acid at the active site is shown in blue:
In this drawing the structure of the active site can be clearly determined, including the 6-carbon ring.
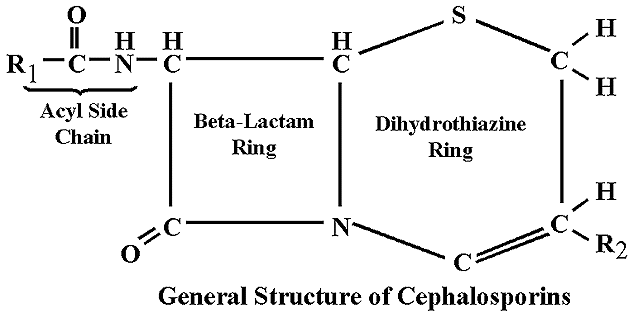
Beta-lactamase is used as a catalyst for the hydrolysis of depsipeptides. This allows the beta-lactamase to destroy the beta-lactam ring in penicillins and cephalosporins.
The reactions of beta-lactamase do not have to be catalyzed, and occur in the presence of any beta-lactam ring. Beta-lactamase consists of a series of 265 amino acids, and the Protein Data Bank reported the molecular/structural weight to be 30077.55 but does not give the units.
Sources:
Medical Dictionary
Photo #1
Photo #2
Photo #3
Structures
Reaction Info.
Photo #4
Protein Data Bank