Now that the semester has come to a close, I am writing my fourth and final blog post as part of my General Chemistry II class. As I reflect back upon the semester, I remember feeling unmotivated in the last weeks. However, I also learned a very important lesson that even though I may lose my motivation when I receive a blow like a bad quiz or test grade, my character is defined by how I react in those moments. I tried to focus my frustration into my study time, and it payed off in the end.
Overall, the most challenging concept of General Chemistry II was trying to differentiate the chapters which contained similar concepts. For example, one chapter was on Acids/Bases and another, on a different exam, was about Buffer Solutions. Also, a difficult concept to grasp was when to use an ICE table and when not to.
My advice to future Gen. Chem. II students: study and practice problems as much as you can. Also, take advantage of any extra credit opportunities because they can make the difference in a letter grade (or, in some cases, whether you pass or fail.) Lastly, don't sweat the small stuff. Yes, quizzes are hard, but they end up not being a huge part of your grade. Just do your best! :)
Wednesday, May 1, 2013
Wednesday, April 17, 2013
Blog Post #3- Investigating a Question About Buffer Solutions
Recently, a question was posed to me by a classmate:
a) Begin by defining what a buffer is and summarize 3-4 buffer characteristics
b) Solve this problem: A buffer is created by combining 150.0mL of 0.25M HCHO2 with 75.0mL of 0.20M NaOH. Determine the pH of the buffer.
While this question is undoubtedly one I need more practice on, the difficulty level does not seem up to par with the assignment. This same type of problem was given to us in the homework, and on our recent test. Personally, I feel that it is significant due to the fact I struggled with a similar type of question on the exam. However, I doubt that the instructor feels the same way.
a) Begin by defining what a buffer is and summarize 3-4 buffer characteristics
b) Solve this problem: A buffer is created by combining 150.0mL of 0.25M HCHO2 with 75.0mL of 0.20M NaOH. Determine the pH of the buffer.
While this question is undoubtedly one I need more practice on, the difficulty level does not seem up to par with the assignment. This same type of problem was given to us in the homework, and on our recent test. Personally, I feel that it is significant due to the fact I struggled with a similar type of question on the exam. However, I doubt that the instructor feels the same way.
Here is my answer to this question:
a) A buffer is a solution which resists a change in pH by neutralizing an added acid or base. Some buffer characteristics are:
- A buffer contains significant amounts of both a weak acid and its conjugate base. For example, human blood is composed of the weak acid carbonic acid (H2CO3) and its conjugate base (HCO3-).
- When additional base is added to a buffer solution, the weak acid reacts with the base, neutralizing it.
- When additional acid is added to a buffer solution, the conjugate base reacts with the acid, neutralizing it.
- Weak acids and conjugate bases by themselves do not contain sufficient bases or acids respectively to work as neutralizing/buffering agents.
b) In order to solve this problem, I must first determine the amount of the weak acid that is converted to a conjugate base by the addition of the NaOH. This is found by comparing stoichiometric ratios presented in the equation:
Once we have determined the amount of HCHO2 present in equilibrium, we can use the concentrations of the weak acid and the conjucate base, along with the pKa value which will be plugged into the Henderson-Hasselbalch equation in order to find the pH of the buffer solution.The Henderson-Hasselbalch equation can be used because the "x is small approximation" is relevant in this context due to the fact that the initial concentration of weak acid is much larger than its Ka value.
HCHO2+NaOH→
H2O+NaCHO2
From this equation, we can then set up a table to track the changes induced by the addition of the NaOH. Please note that the role of water in this reaction does not effect the outcome of our problem and thus has been omitted:
HCHO2
|
NaOH
|
NaCHO2
|
|
Before
Addition
|
0.0375mol
|
0mol
|
0mol
|
Addition
|
0mol
|
0.015mol
|
0mol
|
After
Addition
|
0.0225mol
|
≈0mol
|
0.015mol
|
Once we have determined the amount of HCHO2 present in equilibrium, we can use the concentrations of the weak acid and the conjucate base, along with the pKa value which will be plugged into the Henderson-Hasselbalch equation in order to find the pH of the buffer solution.The Henderson-Hasselbalch equation can be used because the "x is small approximation" is relevant in this context due to the fact that the initial concentration of weak acid is much larger than its Ka value.
The Henderson-Hasselbalch equation is as follows:
pH=pKa+log[base] /[acid]
The pKa was determined by using the Ka value, 1.8x10-4 in this equation:
pKa=-logKa
This value (3.7447), along with the amounts of the weak acid (0.0225mol) and its conjugate base(0.015mol), were plugged in to the Henderson-Hasselbach equation, leaving us with this expression:
pH=3.7447+log(0.015/0.0225)
Solved algebraically, we are left with:
pH=3.57
My reference for this problem was:
Tro, Nivaldo J. Chemistry: A Molecular Approach. 2011. Pages 667,714-715, 722-723
Tro, Nivaldo J. Chemistry: A Molecular Approach. 2011. Pages 667,714-715, 722-723
Thursday, March 14, 2013
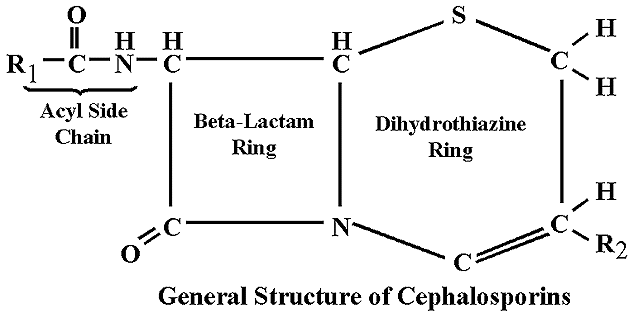
Beta-Lactamase
Beta-lactamase is any member of a group of enzymes which break down penicillins and cephalosporins and destroy their antibiotic activity. These enzymes are produced by gram-negative bacteria, and allow them to be antibiotic resistant. The following image summarizes the effects of beta-lactamase on penicillin:
This is the structure of the Beta-lactamase enzyme itself:
Here is another rendering of the molecule, from the bacteria Francissela tularensis, the inhibitor at the active site of the molecule, is shown in blue.
Here is a related structure in a bacteria that is commonly known, Escherichia coli, once again, the inhibiting acid at the active site is shown in blue:
In this drawing the structure of the active site can be clearly determined, including the 6-carbon ring.
Beta-lactamase is used as a catalyst for the hydrolysis of depsipeptides. This allows the beta-lactamase to destroy the beta-lactam ring in penicillins and cephalosporins.
The reactions of beta-lactamase do not have to be catalyzed, and occur in the presence of any beta-lactam ring. Beta-lactamase consists of a series of 265 amino acids, and the Protein Data Bank reported the molecular/structural weight to be 30077.55 but does not give the units.
Sources:
Medical Dictionary
Photo #1
Photo #2
Photo #3
Structures
Reaction Info.
Photo #4
Protein Data Bank
This is the structure of the Beta-lactamase enzyme itself:
Here is another rendering of the molecule, from the bacteria Francissela tularensis, the inhibitor at the active site of the molecule, is shown in blue.
Here is a related structure in a bacteria that is commonly known, Escherichia coli, once again, the inhibiting acid at the active site is shown in blue:
In this drawing the structure of the active site can be clearly determined, including the 6-carbon ring.
Beta-lactamase is used as a catalyst for the hydrolysis of depsipeptides. This allows the beta-lactamase to destroy the beta-lactam ring in penicillins and cephalosporins.
The reactions of beta-lactamase do not have to be catalyzed, and occur in the presence of any beta-lactam ring. Beta-lactamase consists of a series of 265 amino acids, and the Protein Data Bank reported the molecular/structural weight to be 30077.55 but does not give the units.
Sources:
Medical Dictionary
Photo #1
Photo #2
Photo #3
Structures
Reaction Info.
Photo #4
Protein Data Bank
Friday, February 8, 2013
Tryptophan
Recently, we have been discussing organic molecules, and each student in the class chose an organic molecule to research. I chose tryptophan.
Many of you may know tryptophan as "the thing that makes you sleepy when you eat turkey."
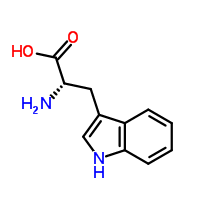
However, tryptophan looks more like this,
Than this:
In fact, the correct (IUPAC) name for tryptophan is (2S)-2-amino-3-(1H-indol-3-yl)propanoic acid. (And I thought tryptophan was a mouth-full!) Tryptophan is one of the 20 different kinds of amino acids (monomers that combine to form proteins). It is also an essential amino acid, which means it can't be synthesized in the body and must be consumed (in forms like turkey).
The chemical formula: C11H12N2O2 gives tryptophan a molecular weight of a whopping 204.225 grams per mole! Tryptophan is soluble in water, boils at a temperature of 447.908 °C, and melts at 280-285 °C. Tryptophan also contains hydrogen bonds, which means that there are also dispersion and dipole-dipole intermolecular forces.
So maybe, the next time you eat a bite of this:
Many of you may know tryptophan as "the thing that makes you sleepy when you eat turkey."
However, tryptophan looks more like this,
In fact, the correct (IUPAC) name for tryptophan is (2S)-2-amino-3-(1H-indol-3-yl)propanoic acid. (And I thought tryptophan was a mouth-full!) Tryptophan is one of the 20 different kinds of amino acids (monomers that combine to form proteins). It is also an essential amino acid, which means it can't be synthesized in the body and must be consumed (in forms like turkey).
 |
| Good news for this guy, huh? |
The chemical formula: C11H12N2O2 gives tryptophan a molecular weight of a whopping 204.225 grams per mole! Tryptophan is soluble in water, boils at a temperature of 447.908 °C, and melts at 280-285 °C. Tryptophan also contains hydrogen bonds, which means that there are also dispersion and dipole-dipole intermolecular forces.
So maybe, the next time you eat a bite of this:
You'll think more about our friend, tryptophan, than "just the stuff that makes you sleepy."
If you want to learn more about tryptophan, this was the source I used. The same website has a link to a 3D molecular structure!
Subscribe to:
Posts (Atom)